Abstract
The increase of DNA damage and the alteration of the DNA damage response (DDR) are critical features of genetic instability presumably implicated in the pathogenesis of monoclonal B-cell lymphocytosis (MBL) and chronic lymphocytic leukemia (CLL). Immunofluorescence microscopy of γH2AX and 53BP1 was performed for the analysis of DNA double-strand breaks (DSB) in CD19+ lymphocytes of 6 healthy individuals, 6 MBL patients, 21 CLL patients and in cells of the CLL cell line HG-3. In addition, immunoblotting of p-ATM, p-ATR, p-CHK1, p-CHK2 and p-TP53 was used for the analysis of the DNA damage response (DDR). As compared to the γH2AX foci levels in healthy individuals (0.1 focus per CD19+ lymphocyte ± 0.0; mean ± standard error of mean) increased γH2AX foci levels were detected in MBL patients (0.3 focus per CD19+ lymphocyte ± 0.1), CLL patients (1.3 foci per CD19+ lymphocyte ± 0.3) and in cells of the cell line HG-3 (1.0 focus per cell). Particularly high γH2AX foci levels were observed in immunoglobulin heavy-chain variable-region (IGHV)-unmutated, 11q-deficient, 17p-deficient and TP53 -mutated CLL. Co-localization of γH2AX and 53BP1 indicated promotion of 53BP1-mediated nonhomologous end-joining (NHEJ) repair mechanisms at sites of DSB. Absent expression of p-CHK1 and p-TP53 suggested impaired G2/M checkpoint control and impaired apoptosis signaling in MBL and CLL. In summary, our results provide evidence for a continuous increase of DNA damage across the spectrum from MBL to CLL in conjunction with an impaired DDR. Particularly high γH2AX foci levels were detected in IGHV -unmutated, 11q-deficient, 17p-deficient and TP53 -mutated CLL. Moreover, abundant γH2AX/53BP1 foci suggested a critical role for (in)effective NHEJ repair mechanisms at sites of DSB in MBL and CLL.
Figure legends
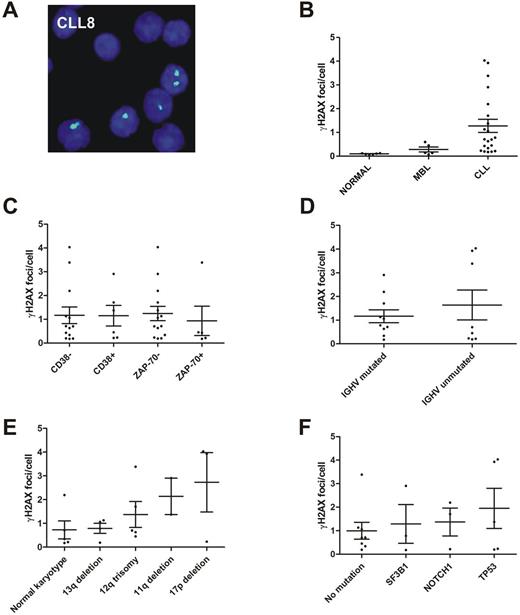
Fig. 1. Analysis of γH2AX foci in MBL and CLL patient samples. (A) Fluorescence microscopic image of γH2AX foci (green, Alexa 488) in nuclei (blue, DAPI) of the sample CLL8. (B) γH2AX foci levels in CD19+ lymphocytes of healthy individuals, MBL and CLL patients. (C) γH2AX foci levels in CLL samples according to CD38 and ZAP-70 expression. (D) γH2AX foci levels in CLL samples according to the immunoglobulin heavy-chain variable-region mutational status. (E) γH2AX foci levels according to cytogenetic risk categories. The categories were defined by (1) harboring a normal karyotype, (2) a 13q deletion as sole aberration, (3) a 12q trisomy but not an 11q or 17p deletion, (4) an 11q deletion but not a 17p deletion and (5) a 17p deletion. (F) γH2AX foci levels according to gene mutation categories. The categories were defined (irrespective of the karyotype) by harboring (1) no mutations in SF3B1, NOTCH1 and TP53, (2) a SF3B1 mutation but not a TP53 mutation, (3) a NOTCH1 mutation but not a TP53 or SF3B1 mutation and (4) a TP53 mutation (irrespective of other mutations). Note: Each dot in the graphs B - F represents the mean γH2AX foci level of one sample. MBL, monoclonal B-cell lymphocytosis; CLL, chronic lymphocytic leukemia.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.